SHORT NEWS
Drinking water from fog: researchers capture clean water from polluted air
A specially coated metal mesh can extract water from fog and remove environmental pollutants at the same time. With the technology developed in Zurich, drinking water can be extracted from the air even in regions with heavy air pollution.

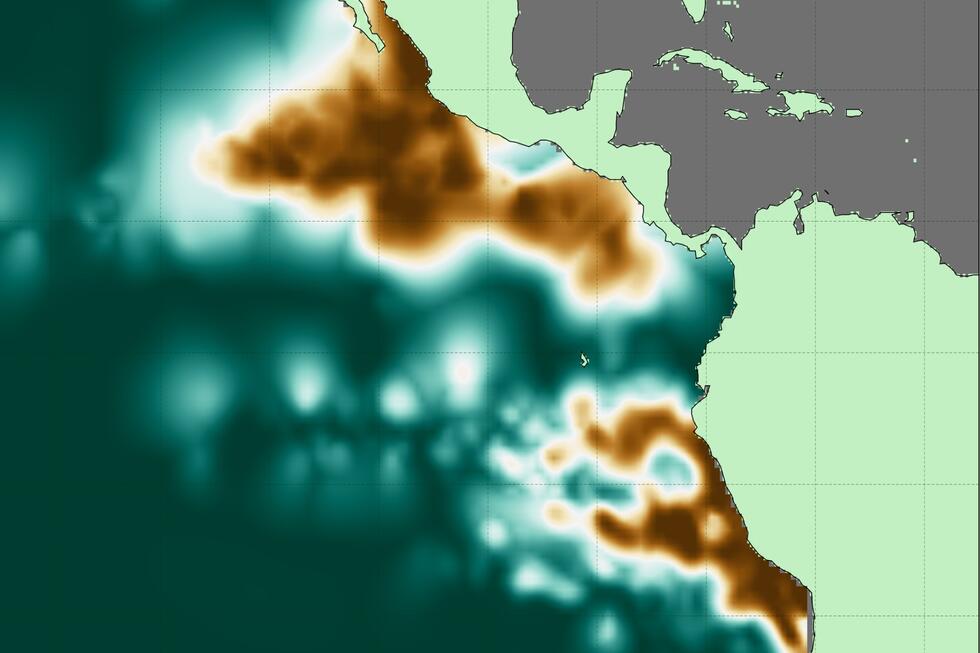
The researchers from ETH Zurich presented the new net in the scientific journal "Nature Sustainability". So-called fog collectors are nothing new in themselves: they are already used in Peru, Bolivia, Chile, Morocco and Oman.
They work according to a simple principle: fine-meshed nets are hung vertically. When the wind blows through, the small droplets of mist stick to the net. Over time, the droplets grow until they are so heavy that gravity pulls them down. There, the water is collected in a trough. Up to several hundred litres of water can be obtained in one day with a fog collector that is only a few square metres in size.
Activated by sunlight
The problem is that dirt particles in the air are captured together with the water. In many of the world's large cities, the air is so polluted that the water extracted from the mist is not clean enough to be used untreated as drinking water or for cooking, according to a statement from ETH Zurich.
The ETH researchers therefore developed a kind of metal net that simultaneously renders the toxic air particles harmless. To do this, they coated the net with polymers and titanium oxide. The polymers ensure the ideal adhesion of the water to the net. The titanium dioxide acts as a chemical catalyst. It breaks down many organic pollutant molecules contained in the droplets, such as diesel droplets or the hormone-active chemical bisphenol A, and thus makes them harmless.
The titanium oxide's splitting function is activated by sunlight. According to the researchers, it must regularly receive UV light from the sun in order to regenerate. An effect known as photocatalytic memory ensures that half an hour of sunlight is enough for the titanium oxide to remain active for 24 hours. In the future, the researchers would like to use it to recover water from cooling towers, for example.